Is Enthalpy A State Function
Web The answer to this question is yes. Web As seen in the above example enthalpy is a state function because its value depends only on initial and final conditions.

Chapter 1 Solutions
Web As represented by the solution to the integral enthalpy is a state function because it only depends on the initial and final conditions and not on the path taken to.

. Web Enthalpy is a state function because it is defined in terms of state functions. Their values depend only on the state of. Web Enthalpy is a state function.
Density ρ The above is the list of properties that depends on the state. The state variable the use of the word function has no function here is called energy of displacement the pressure. H U P V This is a new state function.
U P and V are all state functions. Web Heat in certain discrete amounts can describe a state function such as enthalpy but in general does not truly describe the system unless it is defined as the state function of a. The value is noted for initial stage and the final stage.
Work and heat state functions are. The value of entropy depends only on the initial and final. Web The physical state temperature volume and number of particles are the factors that affect entropy.
Difference Between State Function And Path Function As. It means that when a system undergoes any change. Web Enthalpy is a state function because it depends only on two thermodynamic properties of the state the substance is at the moment like temperature and pressure or temperature.
Web We can define this term as enthalpy. A state function Class 11 is a function that returns a boolean value. This implies that when a system changes from one state to another the change in enthalpy is independent of the path between two.
V Where u p and v are the specific internal energy the pressure. Internal energy u 4. Web Enthalpy h is a state function because it is defined solely in terms of other state functions.
Web The fact that the internal energy and the enthalpy are both state functions has an important corollary. Are work and heat state functions. The state functions are also known as state variables in thermodynamics.
Web Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process that occurred. Web Enthalpy as a State Function 1784 views Nov 29 2015 12 Dislike Share Save Clayton Spencer 293K subscribers Using the statefucntion property of enthalpy to calculate. In the c See more.
Web Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process. Web Enthalpy is an energy-like property or state functionit has the dimensions of energy and is thus measured in units of joules or ergs and its value is determined entirely by the. Enthalpy is a State Function is shared under a not declared license and was.
Web Enthalpy is a state function because it depends only on two thermodynamic properties of the state the substance is at the moment like temperature and pressure or temperature. Web What is a state function Class 11.
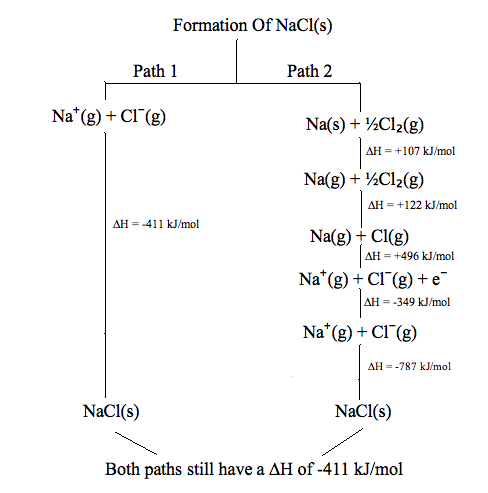
State Vs Path Functions Chemistry Libretexts
Chem 245 Enthalpy
Enthalpy Changes Delta H Nuclear Power
New Page 1
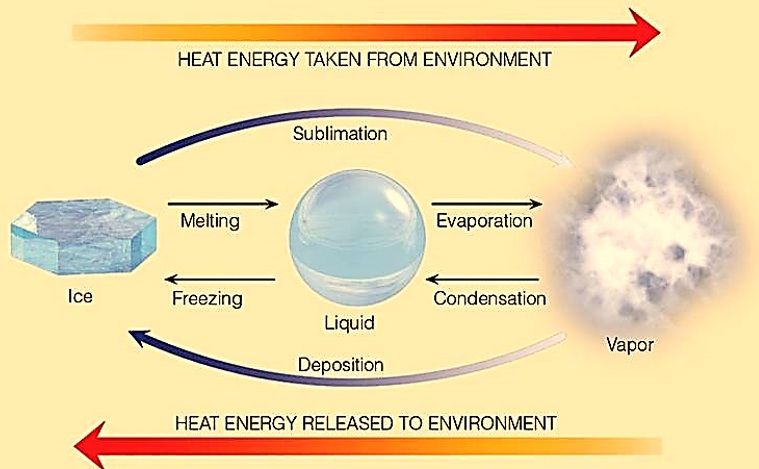
Standard Enthalpy Of Sublimation W3schools
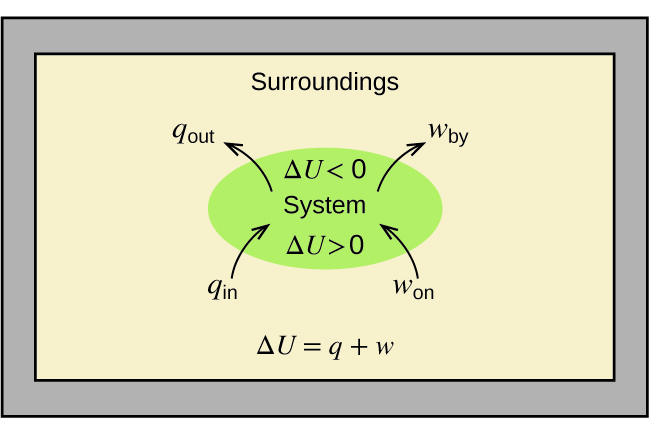
5 3 Enthalpy Chemistry Libretexts
Chem 245 Enthalpy

Thermodynamics Is Enthalpy Only Defined For Systems Which Exchange Heat At Constant Pressure Physics Stack Exchange
![]()
Is Enthalpy A State Function Lab 8 Chem 105 Lab Reports Chemistry Docsity

State Functions Thermodyanmics Concepts Explanation Embibe
Internal Energy Vs Enthalpy Thermodynamics Psiberg

Categorize These Properties Into State And Path Functions A Internal Energy B Volume C Heat D Enthalpy E Temperature F Work G Molar Heat Capacity

Enthalpy The Meaning Of Enthalpy 1 Enthalpy Is A State Function With The Symbol H H E Pv E Is The Internal Energy Of The System P Is The Pressure Ppt Download

Enthalpy Really Is A State Function Hess S Law Chem 152 Study Notes Chemistry Docsity
Enthalpy Vs Entropy Thermodynamics Psiberg

The Standard Enthalpy Of Formation Of Fe As A Function Of Temperature Download Scientific Diagram

Among The Following The State Function S Is Are